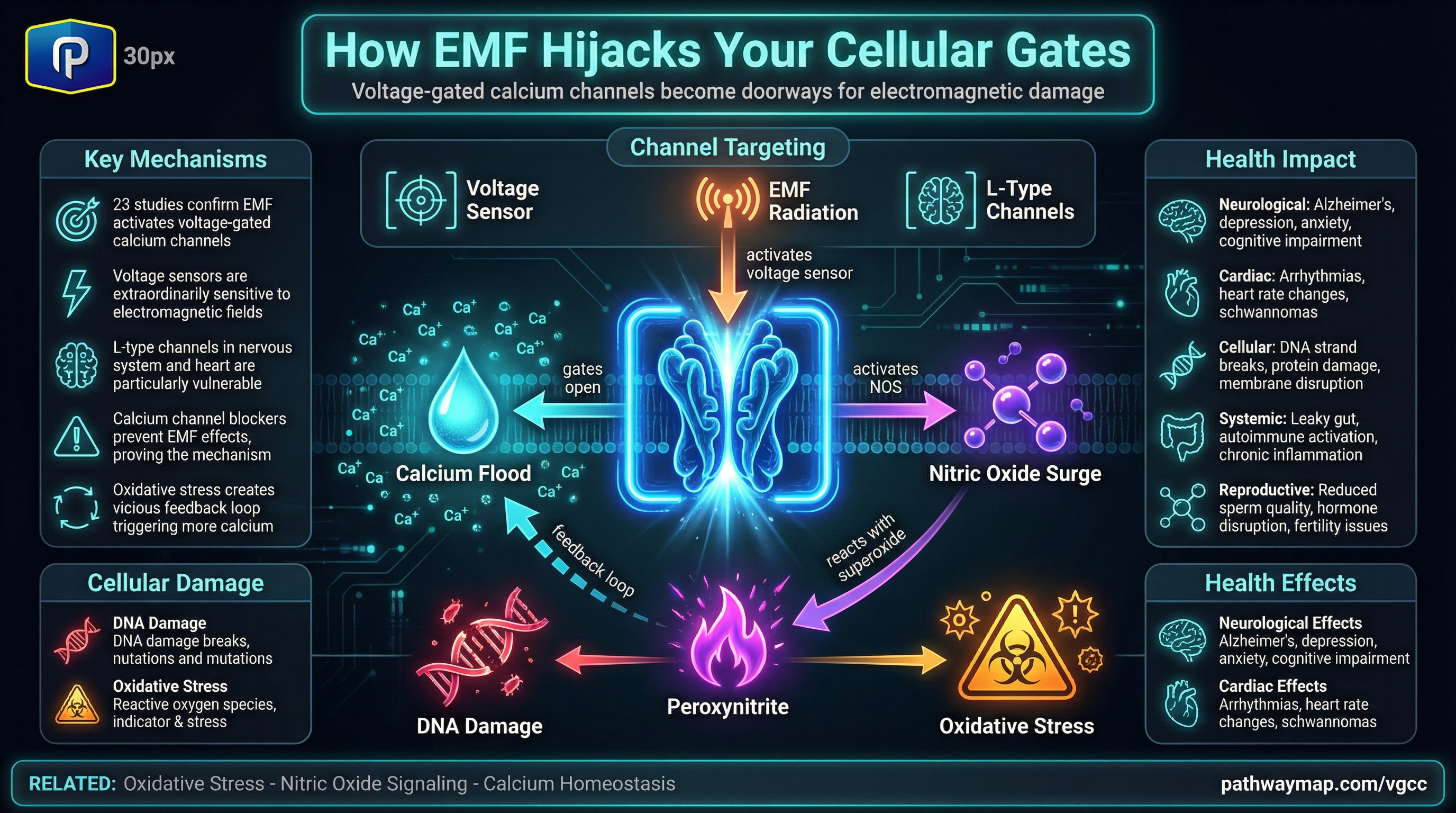
The relationship between MTHFR mutations and folic acid metabolism represents one of the most nuanced topics in nutritional biochemistry. While it’s true that MTHFR mutations don’t prevent folic acid processing, the full story reveals legitimate concerns about synthetic folic acid that deserve careful consideration.
The distinct roles of DHFR and MTHFR
You’re correct that DHFR (dihydrofolate reductase) is the enzyme responsible for converting folic acid, not MTHFR. This critical distinction helps clarify the biochemical pathway:
- Folic acid (synthetic) enters the system through fortified foods or supplements
- DHFR converts folic acid → dihydrofolate → tetrahydrofolate (THF)
- THF undergoes various transformations to 5,10-methylenetetrahydrofolate
- MTHFR converts 5,10-methylenetetrahydrofolate → 5-methyltetrahydrofolate (5-MTHF)
However, here’s where it gets problematic: DHFR activity in humans is extremely limited. Research published in PNAS found that human liver DHFR has less than 2% of the activity found in rats. This means our capacity to process synthetic folic acid is inherently poor, regardless of MTHFR status.
Key finding: Doses of folic acid as low as 200mcg can lead to unmetabolized folic acid (UMFA) in the bloodstream, yet a single serving of fortified breakfast cereal can contain up to 400mcg.
The UMFA problem: More than just inefficiency
When DHFR becomes saturated—which happens easily given its poor activity—unmetabolized folic acid accumulates in the blood. Studies show that 38% of older adults have detectable UMFA, with an average representing about 6% of their total serum folate.
The concerns about UMFA are substantial and evidence-based:
- Receptor competition: UMFA competes with natural 5-MTHF for folate transporters and receptors, potentially creating a functional folate deficiency despite adequate intake
- Immune dysfunction: UMFA may inhibit MAIT cells and natural killer cells, potentially affecting immune surveillance and cancer prevention
- Masking B12 deficiency: High folic acid can mask megaloblastic anemia from B12 deficiency, allowing neurological damage to progress undetected
- Potential fibrosis: Recent research suggests UMFA may promote organ fibrosis through folate receptor signaling
MTHFR mutations compound the problem
While people with MTHFR mutations can technically process folic acid, the reality is more complex. The C677T variant reduces MTHFR activity by 35-70%, creating a bottleneck in the methylation cycle. When combined with DHFR’s already poor function, this creates multiple points of impairment.
Research shows this can lead to what’s called “pseudo-MTHFR syndrome” even in people without MTHFR mutations. High doses of folic acid can paradoxically increase homocysteine levels—the opposite of what we want from folate supplementation.
The binding affinity problem
Here’s a crucial finding that explains why folic acid can be problematic: Research shows that folic acid has the highest binding affinity for all folate receptors, followed by 5-MTHF, then folinic acid. This means folic acid preferentially occupies folate receptors, potentially blocking the transport and utilization of active folates.
As one researcher noted, folic acid can “bind or block the natural 5-MTHF receptors and transporters”, creating a situation where despite adequate folate intake, cells may be functionally deficient in usable folate.
Why medical organizations still recommend folic acid
Despite these concerns, organizations like the CDC maintain that people with MTHFR variants can process folic acid and should take it to prevent neural tube defects. This position reflects several realities:
- Folic acid fortification has successfully reduced neural tube defects by 19-32%
- It’s the only form with decades of safety data for pregnancy
- It’s inexpensive and stable in food fortification
- The benefits for preventing birth defects are considered to outweigh potential risks
However, this doesn’t mean folic acid is optimal or without problems. The medical consensus often lags behind emerging research, particularly when public health policies are involved.
The evolutionary perspective: Why MTHFR variants persist
The high frequency of MTHFR variants (40-60% of populations) suggests they’re not simply defects. Research links MTHFR variant frequency to UV radiation exposure, with higher rates in populations from high-UV environments.
These variants may have provided advantages including:
- Enhanced DNA synthesis quality
- Protection against certain cancers
- Folate conservation during scarcity
- Adaptation to high-folate diets
The problem isn’t the variants themselves—it’s the mismatch between our evolved biology and the modern environment flooded with synthetic folic acid.
Practical implications
Given the evidence, a more nuanced approach to folate supplementation makes sense:
- Consider folinic acid or small amounts of 5-MTHF instead of folic acid: 5-MTHF doesn’t require DHFR conversion, doesn’t create UMFA, and is immediately bioavailable
- Be aware of fortified foods: In countries with mandatory fortification, many people already consume significant folic acid without realizing it
- Support overall methylation: Adequate B12, B6, and riboflavin are crucial for proper folate metabolism. Don’t forget Betaine(TMG), choline, and glycine for the other half of methylation.
- Individual assessment: Those with digestive issues, multiple MTHFR variants, or chronic health conditions may be more susceptible to UMFA accumulation
Conclusion
While it’s technically true that MTHFR mutations don’t prevent folic acid processing, this oversimplifies a complex situation. The real issues are:
- DHFR’s inherently poor activity in humans makes everyone susceptible to UMFA accumulation
- Folic acid can compete with and potentially block active folates
- MTHFR variants compound these problems by creating additional bottlenecks
- The modern food environment provides unprecedented exposure to synthetic folic acid
Rather than dismissing concerns about folic acid as myths, we should recognize that both the benefits (neural tube defect prevention) and the potential risks (UMFA accumulation, receptor competition, immune effects) are real. The key is making informed choices based on individual circumstances rather than following one-size-fits-all recommendations.
As researchers increasingly suggest, it may be time to reconsider whether folinic acid or 5-MTHF should replace folic acid in supplementation and fortification programs—not because people can’t process folic acid at all, but because our bodies process it poorly, and this inefficiency may have consequences we’re only beginning to understand.
Author’s thoughts
Folic acid is not good, it’s oxidized and clingy. We should likely all avoid it 100%. I consume bread products with it sometimes but I’ve rebuilt my cells well enough to play around like this again.
The reason someone with MTHFR might think folic acid impacts them worse is because people with MTHFR are loaded with more toxins than others. And for all we know, the people we see handling folic acid actually have MTHFR and just haven’t filled their toxin bucket yet.
MTHFR is just another symptom of doing this wrong. Continue learning how to human